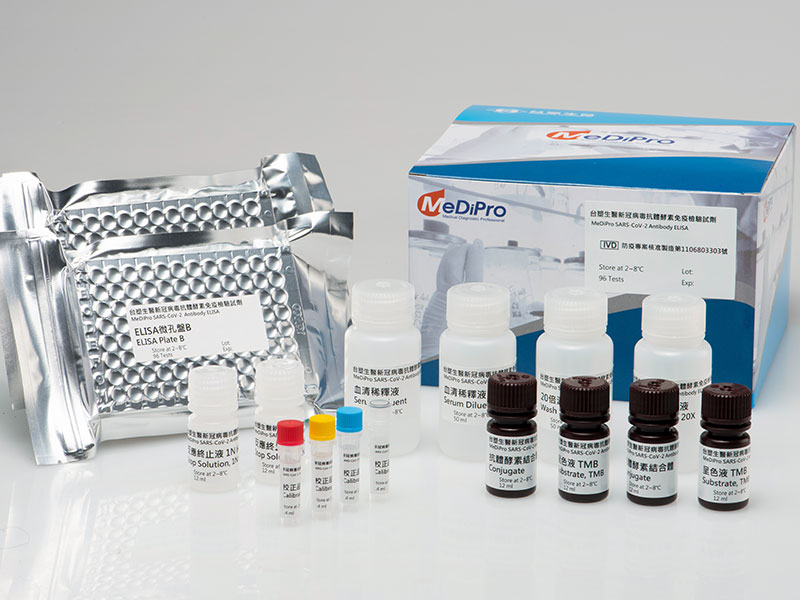
On September 16, 2020, after undertaking the technology of "SARS-CoV-2 Neutralization Antibody Titer Test" jointly developed by Chang Gung University and Chang Gung Memorial Hospital, FBC commenced the development for the optimized conditions for mass production immediately. Following three months of effort, FBC performed three batches of production and arrange the stability test, calibrator development, and clinical performance test (note: compared with traditional virus neutralization test in the BSL3 la-boratory) according to the requirements by TFDA to ensure the ac-curacy of the quantitative testing achieved 90%. Soon, FBC submit-ted a project-based manufacturing application to TFDA. The applica-tion was approved on March 5, 2021. Currently, the production has officially commenced, and FBC has begun the sales planning for Taiwan and international markets.
When the SARS-CoV-2 infects the human body, the immune system will activate the self-protection system and generate antibod-ies. According to Shih Shin-Ru, director of the Chang Gung Univer-sity's Research Center for Emerging Viral Infections, "neutralizing antibodies are favorable antibodies that bond with virus to block its replication and infection; they are the key to the protection of human body after vaccination." The world currently has no answer to whether neutralizing antibodies will be generated after receiving COVID-19 vaccines and how long will the protection last. FBC's "SARS-CoV-2 Neutralization Antibody ELISA" is the best option to address such concern.
According to Steve Liu, Vice President of FBC, in traditional vi-rus neutralization test of approximately three to five days in a P3 la-boratory is required to detect whether sufficient neutralizing antibod-ies are generated after vaccination. The test is time consuming and costly, therefore, no large-scale screening can be arranged, which is less effective in facilitating the opening of border control and the control of the spread of the pandemic. The "SARS-CoV-2 Neutrali-zation Antibody ELISA" developed by Taiwan's virus prevention professor, Shih Shin-Ru , is currently the unique technology in the world that can quantitatively detect neutralizing antibodies. The technology is able to concurrently complete the test for over 90 samples within 1.5 hours. It is the true honor of Taiwan that is wor-thy of our full efforts to promote.
FBC is the largest manufacturer of In Vitro Diagnostic Devices in Taiwan. Its production capacity has reached 3 million tests per month and it has accumulated experience in producing more than 50 million tests of various diagnostic reagents. Chair of FBC, Sandy Wang, instructed the employees to prepare for technology develop-ment and production at the beginning of the Covid-19 outbreak, hoping to fulfill the responsibility as a professional manufacturer of diagnostic tests In Vitro Diagnostic Devices. Through cooperation with the Chang Gung medical group, FBC obtained the project-based manufacturing license for the "SARS-CoV-2 Neutralization Antibody ELISA" at the earliest instance, which will detect the pro-tection of the COVID-19 vaccination and will assist vaccine manu-facturer in Taiwan in development of vaccines. Subsequently, FBC will provide this favorable technology for pandemic control to coun-tries and regions worldwide as soon as possible in the hope of mak-ing contributions to COVID-19 control in Taiwan and around the world.